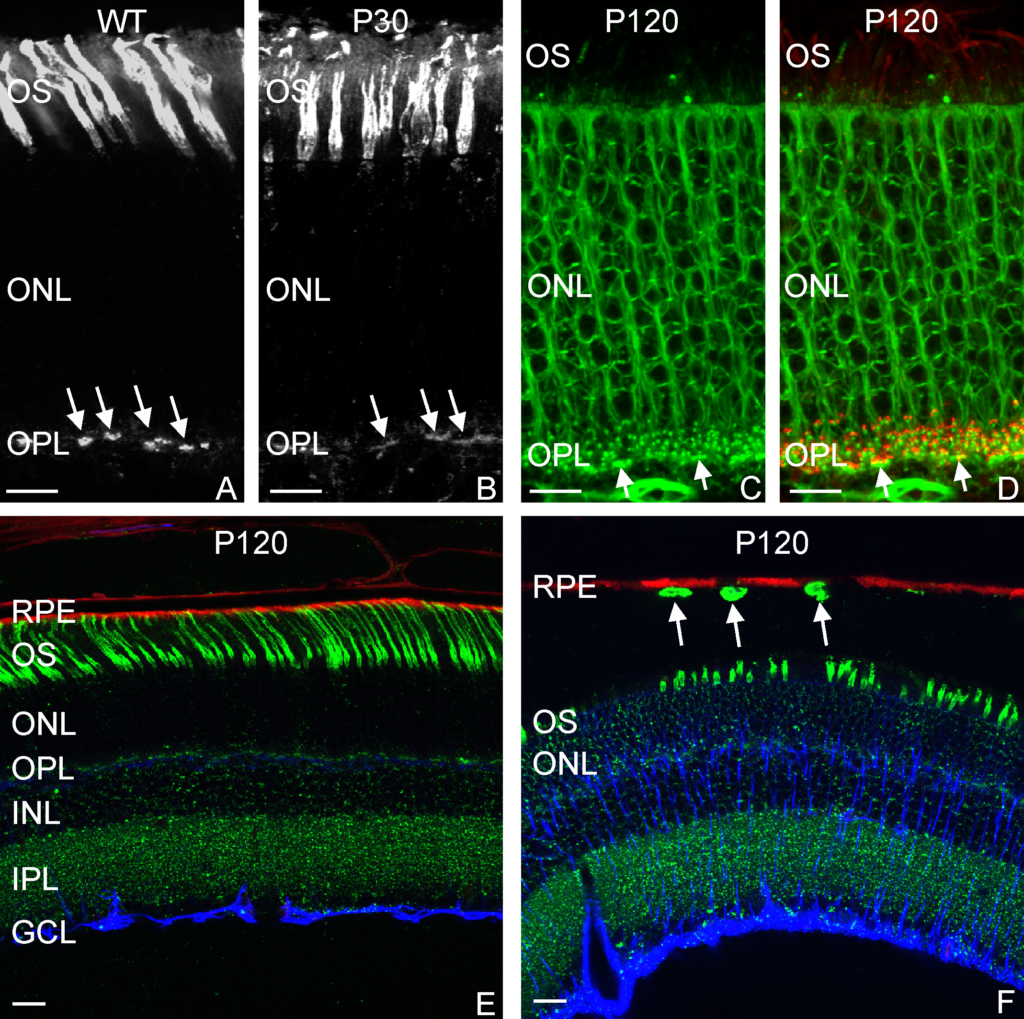
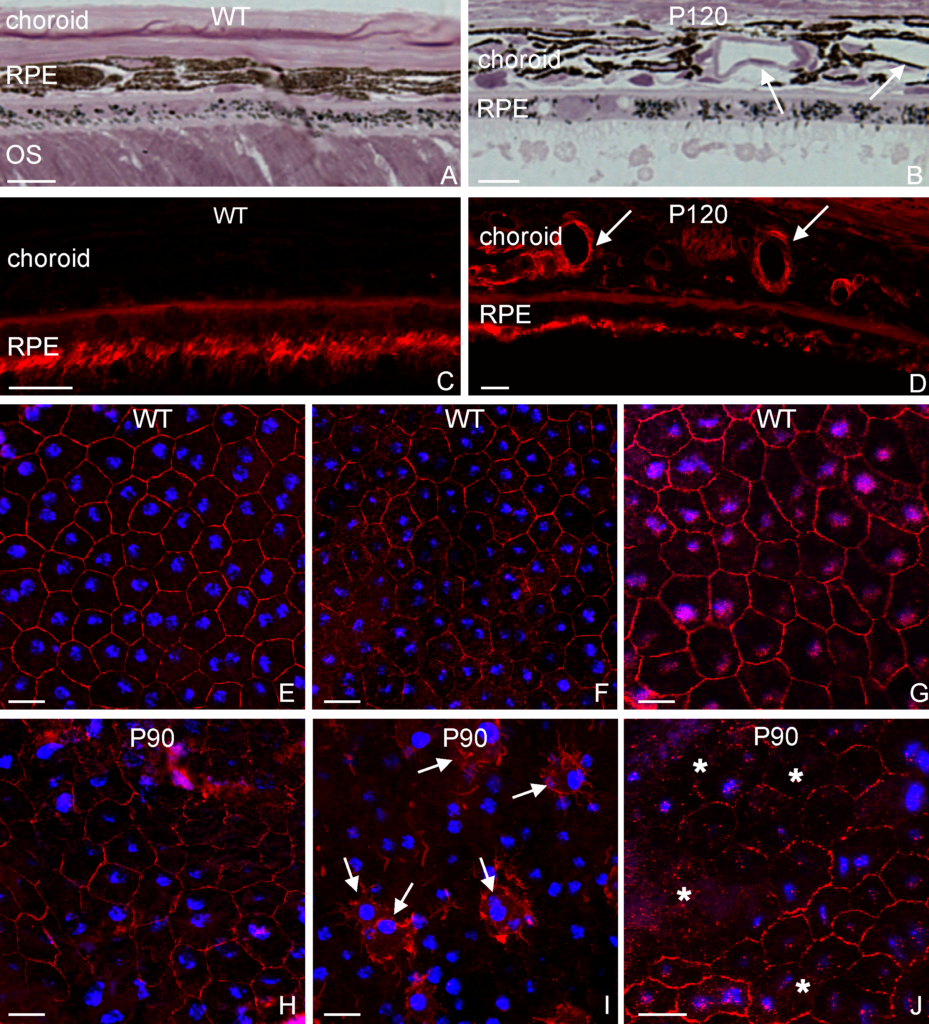
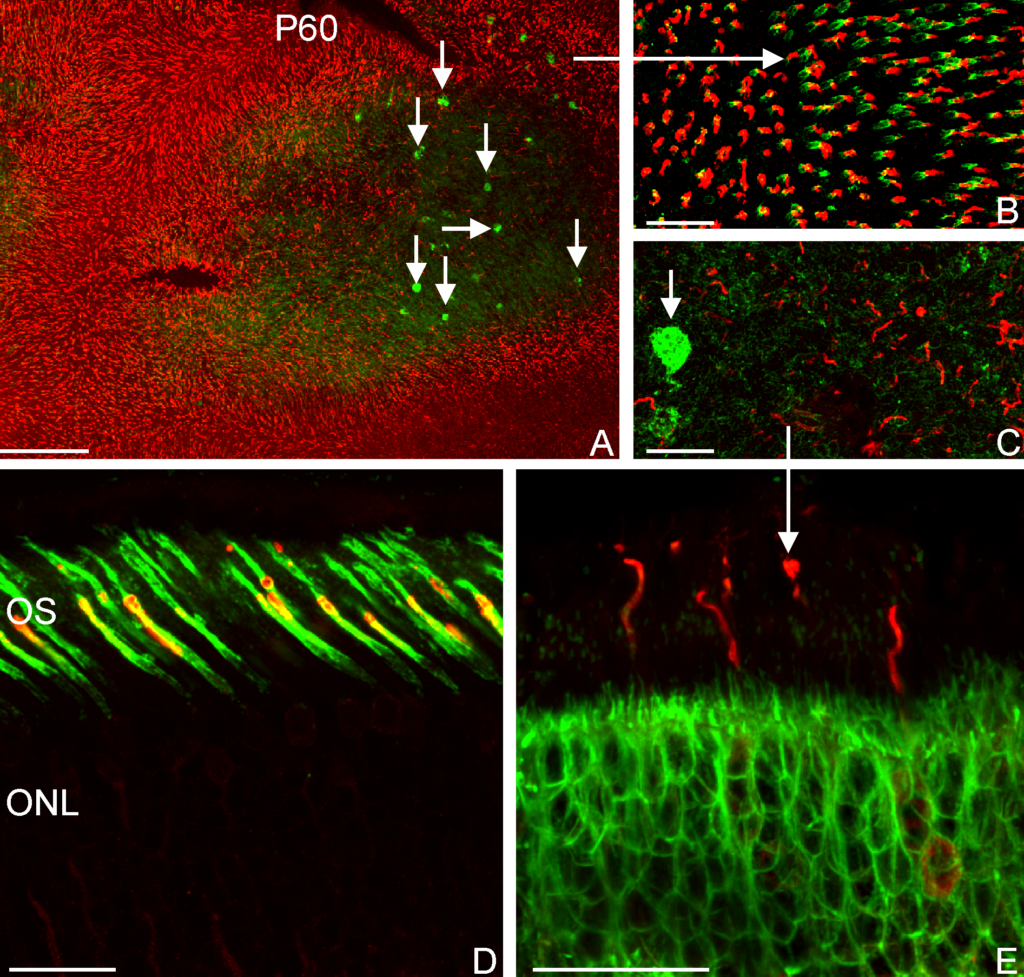
Adipose stem cells (immature fat cells) are among the most abundant cell-type in the human body, developing methodologies that take advantage of these cells for reconstructive procedures may one day provide a source for wound healing as a result of traumatic injury or to ameliorate congenital defects using autologous transplantation, thus obviating the potential of tissue rejection. In a collaboration with Drs. Tracy Clevenger, Steve Fisher, Dennis Clegg we examined the ability of adipose stem cells to breakdown a hydrogel that was engineered to foster their growth and survival as these stem cells differentiated into a more mature state with the long-term rationale of increasing the chance of successful tissue grafting as well as accelerating the process of wound healing. That research was published today in the Journal of Tissue Engineering. This project involved a blend of computer science, molecular and cellular biology, as well as bio-imaging techniques to investigate these complex biological systems.
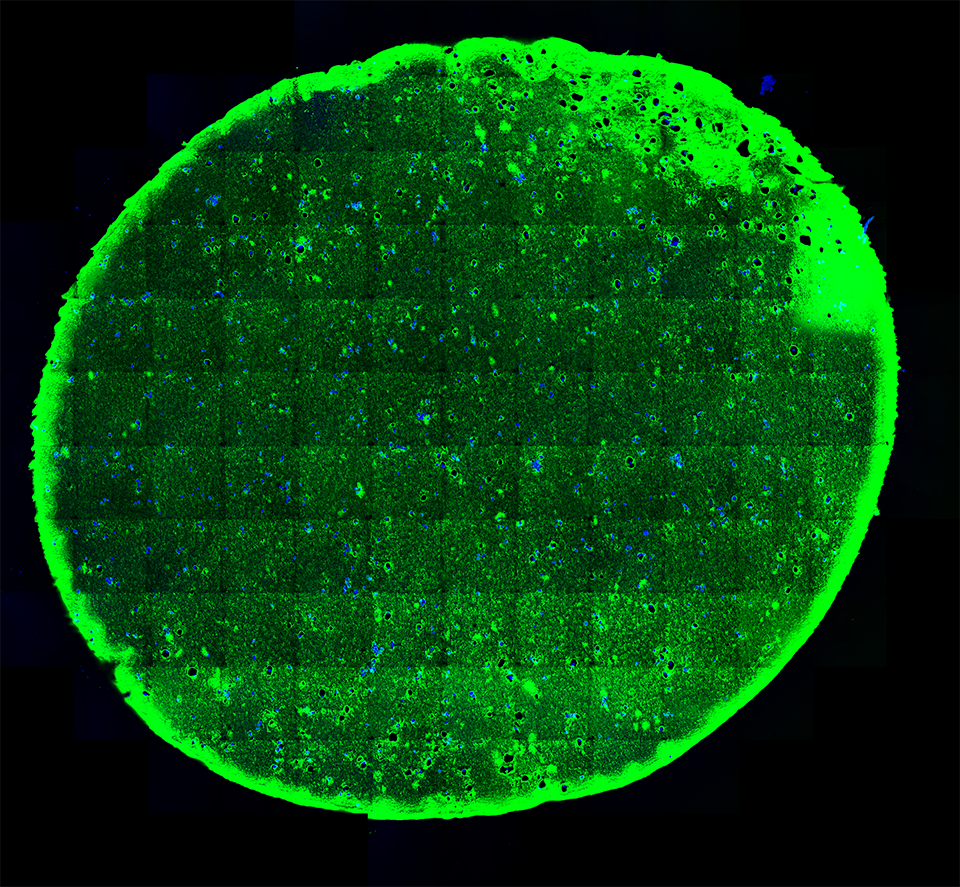
A 20-micron thick slice of a hydrogel immunostained (green; for reference the width of a human hair is about 180-microns) with embedded stem cells (blue) under “normal” conditions visualized using fluorescent microscopy.
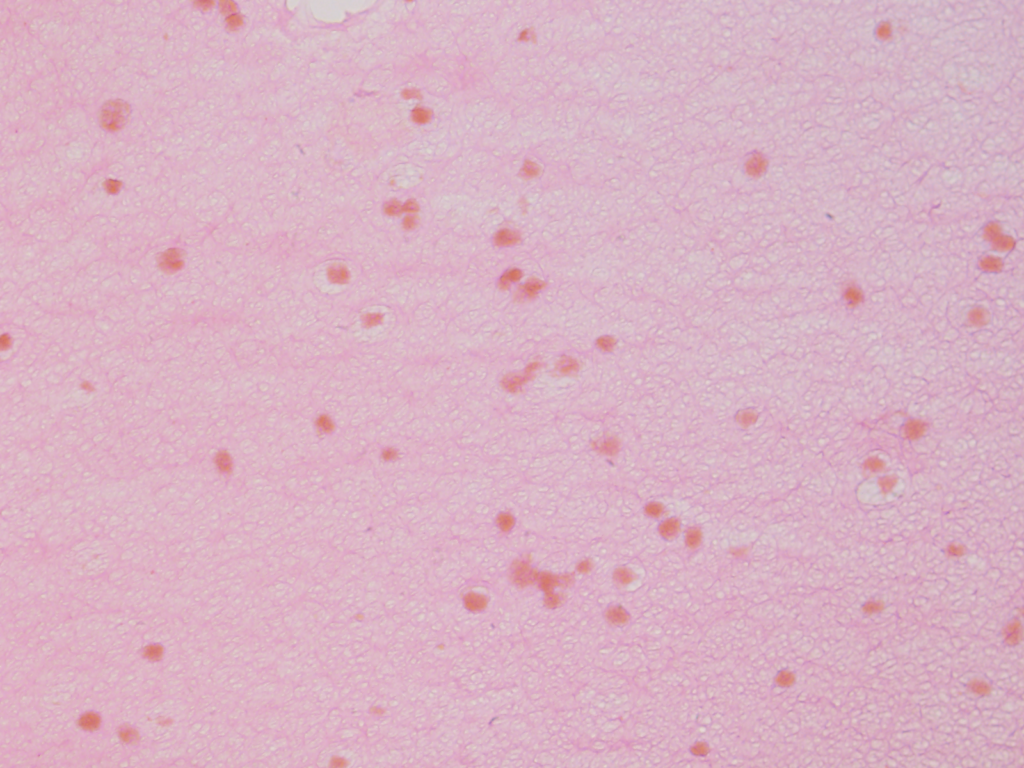
Using a stain that labels eosinophilic structures we are able to visualize the fine processes of hydrogel that uniformly interweave among the adipose stem cells using a light microscope.
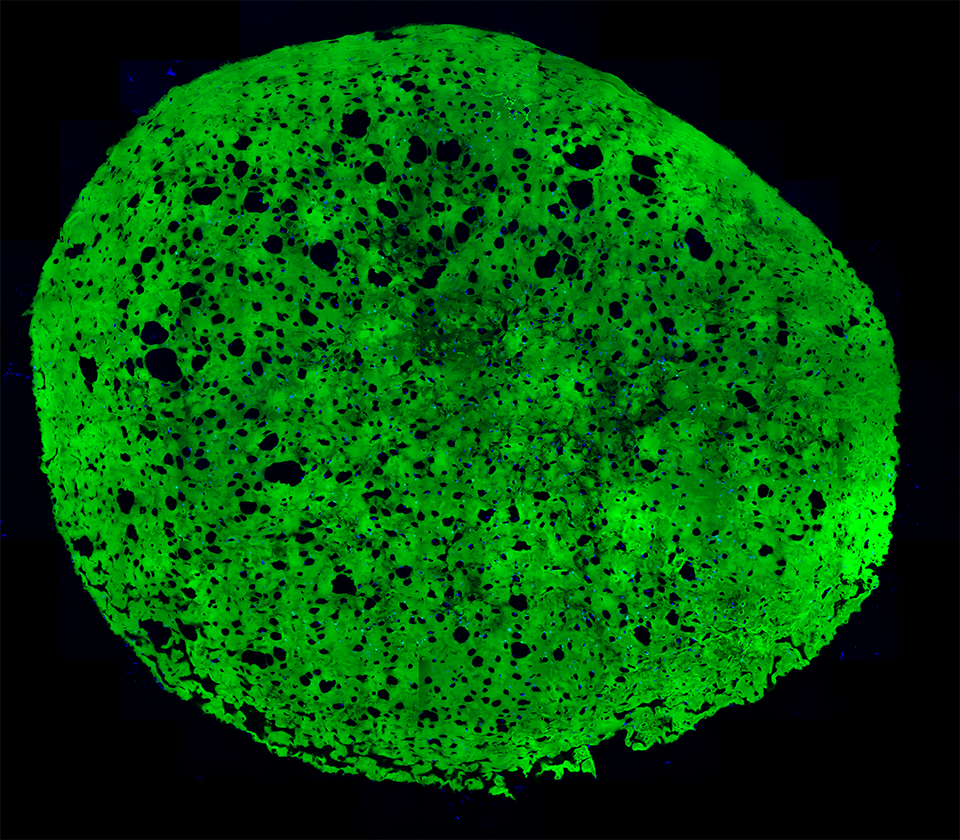
A section of a hydrogel whose embedded stem cells were placed under conditions that induced them to differentiate into more mature state (blue). Notice the increase in the number of cavities after 4 weeks as a result of the adipogenic differentiation.
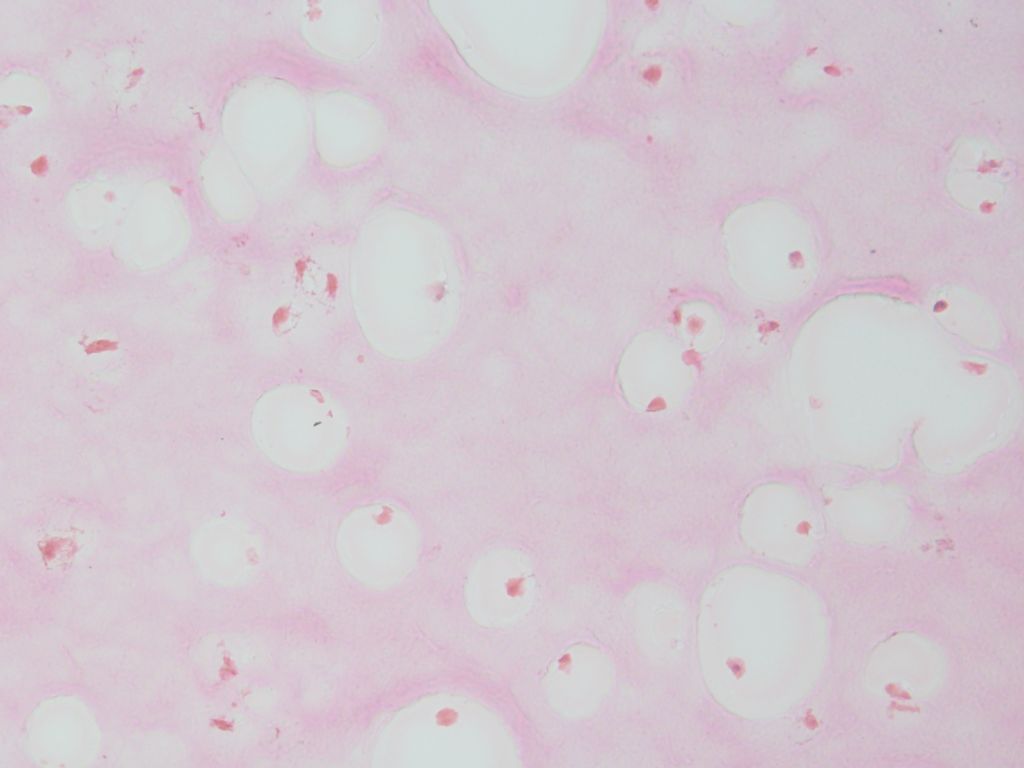
We can also documented the changes in the filamentous appearance of the hydrogel to a increasingly smoother appearance under differentiated conditions, shown in pink.
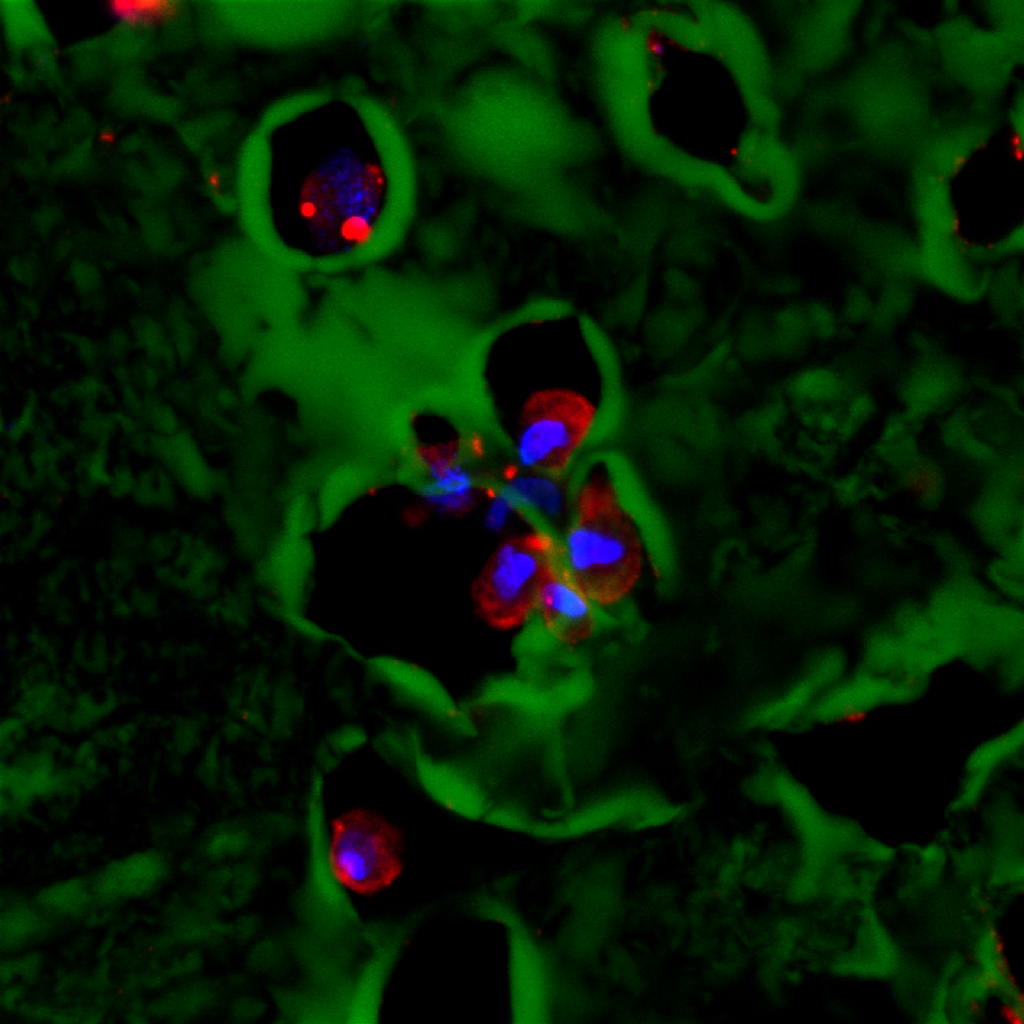
A high-resolution immunofluorescent image of a group of adipose stem cells lining the edges of a subset of cavities within the hydrogel. Here, the gel is visible in green, while the cell nucleus is blue, and the cytoplasm of the cell is red.
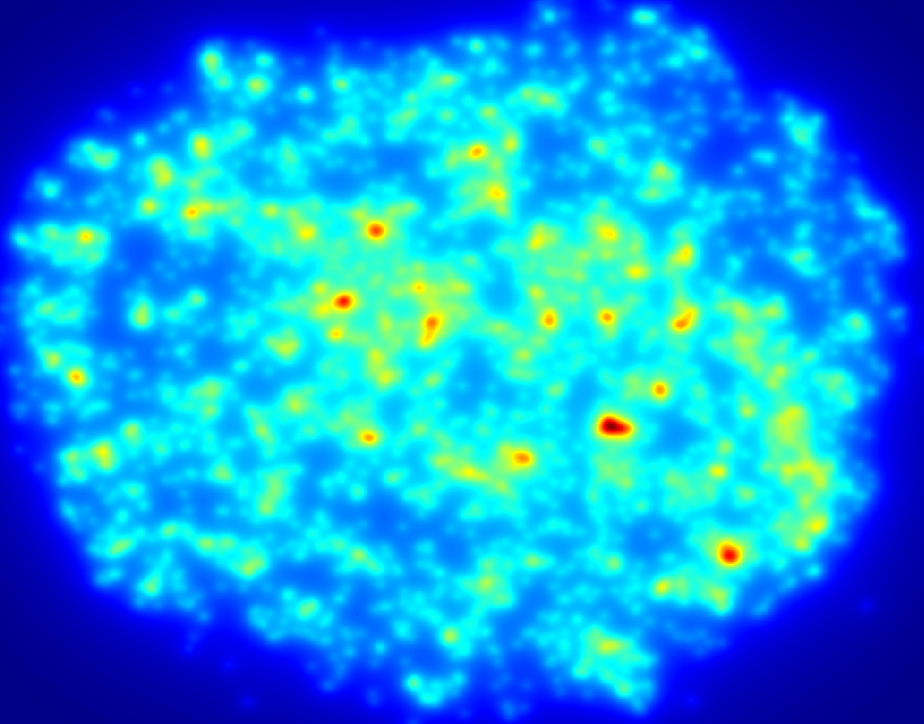
An example of a density map shows the relative distribution of cells across a section of hydrogel, here red areas show more densely populated regions. Data such as this illustrates the importance of undergraduate volunteers who contribute countless hours quantifying various parameters, those efforts help move research along at a faster pace than would otherwise happen.

Dr. Tracy Clevenger, lead author.